A Statement of Intent (SOI) is a preliminary form that researchers complete to outline the key details of their intended research project and their intent to submit a full application for REB review. If your funded research involves human participants at any point, a valid REB number must be provided to Research Support Services (RSS), Strategic Programs and/or the Ontario Agri-Food Innovation Alliance grants team prior to account set-up or release of funds. If you have not yet received REB approval, whether because your project is not yet sufficiently developed to submit to the REB or because REB approval is pending, an approved Statement of Intent is sufficient for the release of funds in advance of receiving REB approval.
Process Overview
When submitting an SOI in EthOS, researchers provide the preliminary details of the intended research project and the Ethics Office completes an administrative review of the SOI application. Rarely, the Ethics Office may request a modification to the SOI content prior to approval.
Key to the process is the anticipated approval date of the subsequent Main REB Application. This anticipated approval date is entered on the SOI application and should account for protocol preparation and review times (as can be best estimated at the time).
If the entered date passes without an REB approval, the SOI must be extended via an SOI Renewal form. Similarly, once REB approval is secured, researchers must officially close out the SOI as part of the grants and funding process. Both actions (renewals and closures) are facilitated by SOI Renewal/Closure forms within EthOS.
To ensure compliance and avoid potential funding access issues, researchers now receive automated system notifications for upcoming SOI expiry:
- Two weeks before expiry
- On the day of expiry
These notifications remind researchers to submit Renewal or Closure forms and update the anticipated REB application approval date, if applicable, before the expiry date. The Ethics Office and the Grants & Contracts team will monitor this process closely due to the funding implications of any expired SOIs.
Create a Statement of Intent
Creating a Statement of Intent is a relatively quick process using a short form. However, remember to save your application periodically especially if you are not able to complete the form in one sitting. EthOS will time out after 30 minutes of inactivity and you could lose any unsaved form content.
To create a Statement of Intent application:
-
From your EthOS Work Area, create a Project
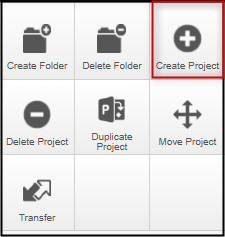
-
Select the Statement of Intent form from the drop-down list and click on the Create button
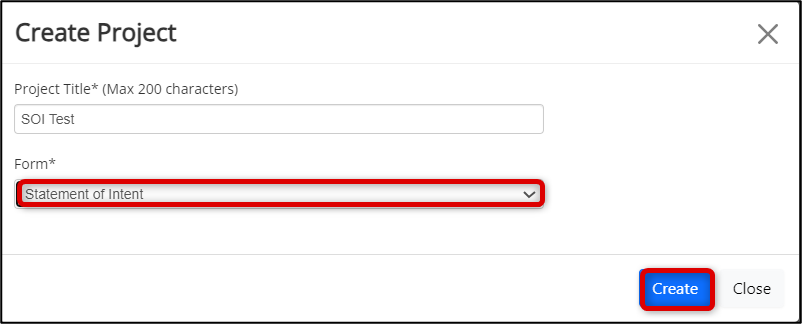
-
The SOI is a short application form with only one section and one page; to get started, click on the Statement of Intent questions link to enter the required details
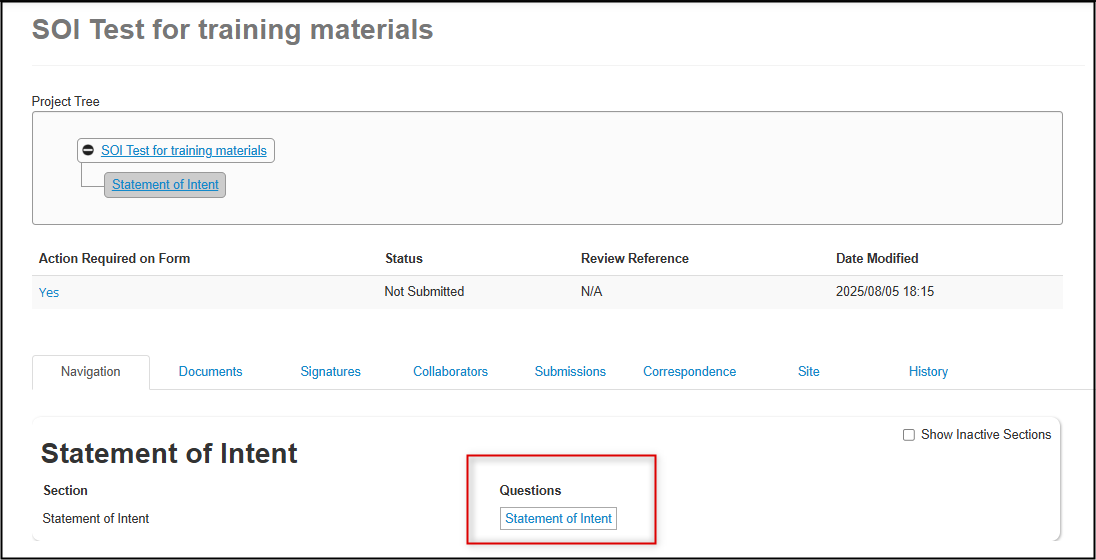
-
First enter the Project Title as an abridged version (for system and display purposes), followed by the complete study title you wish to appear on the SOI approval certificate
- Note: for the short version of the Project Title, if you plan to use the same name for your main REB application, add “SOI” at the beginning. EthOS will not allow you to re-use the same Project Title later on.
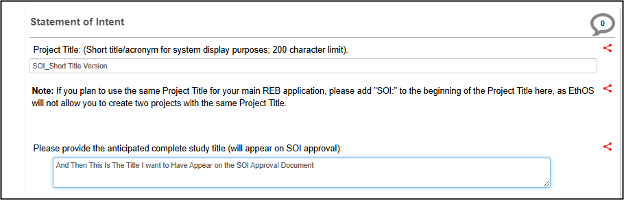
-
Enter the Principal Investigator details
- If you are not the PI, select No, enter the PI name using the Search User box, and proceed to Step 6 of these instructions
-
If you are the PI, select Yes, enter your name using the Search User box, and proceed to Step 7 of these instructions
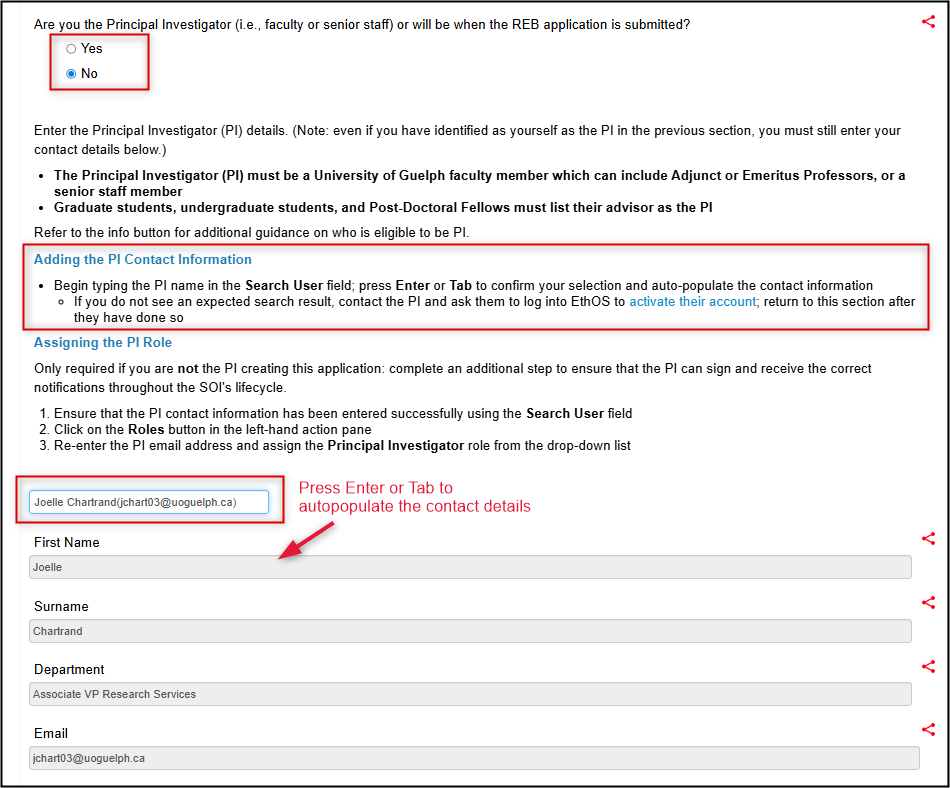
-
Assign the correct access to the PI:
- Click on the Roles button in the left-hand action pane to open the Share Roles pop-up window
- Re-enter the PI email address and choose the Principal Investigator role from drop-down list
-
Click on the green Share Role button and EthOS will display a green Form Shared Successfully banner message
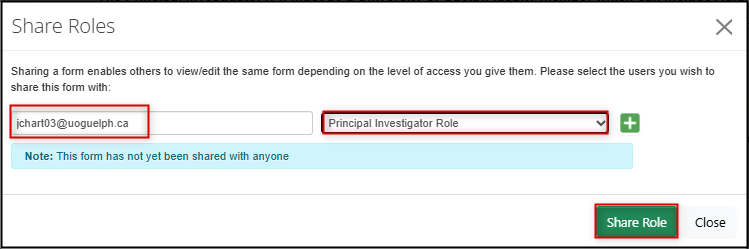
Tip: see the Sharing Forms (Assigning Roles) training material for more guidance on form permissions if needed.
-
While it is not necessary to list all potential team members at this time, you are given the option to do so but must enter your own name if you are not the PI:
-
If you are the PI and have no other study team members to add, select No and proceed to Step 9 of these instructions
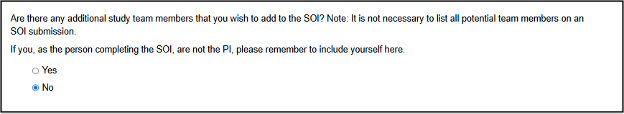
Otherwise, to add yourself or any other study team members, select Yes and enter their names in the expanded section, clicking the green Add Another button until all members are listed
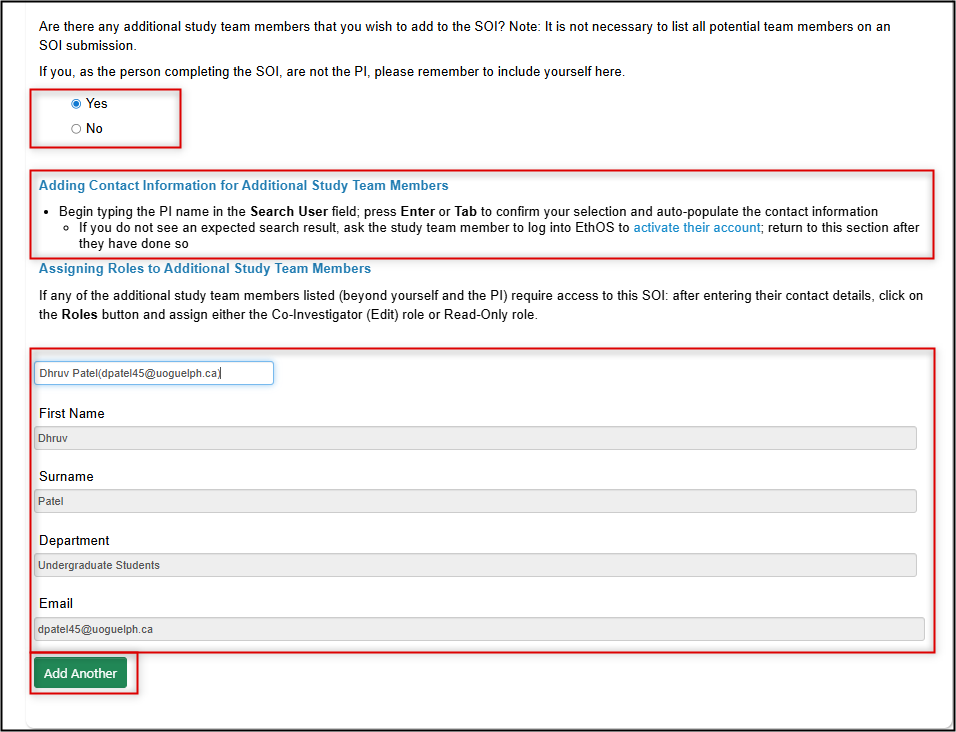
-
-
Optional: Optional: for any additional study team members listed, assign access to the SOI application using an EthOS role (see Step 6 and choose either the Co-Investigator or Read-Only role)
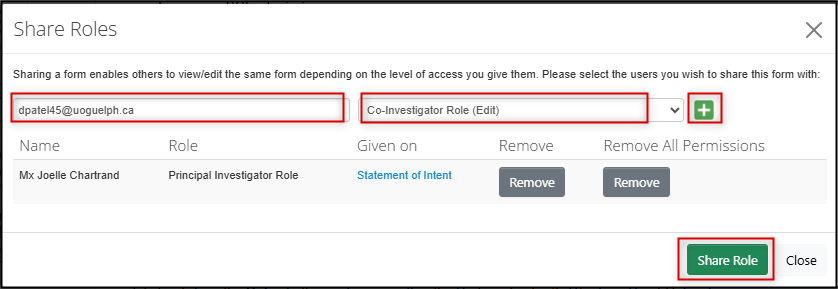
- Click on the Roles button, enter the study team member's email address, and choose the appropriate role from the drop-down list
- Click on the green + button assign roles to more study team members
-
Click on the green Share Role button and EthOS will display a Form Shared Successfully banner message
Tip: see the Sharing Forms (Assigning Roles) training material for more guidance on form permissions if needed.
-
Enter the award and funding details

-
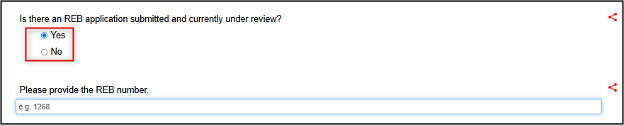
Confirm if there is an active REB application submitted and under review
- If No, proceed to Step 11 of these instructions
- Otherwise enter Yes and complete the additional fields:
- Note: for EthOS-generated applications, the REB number is the 4-digit Project ID reference beside the title in your project list
-
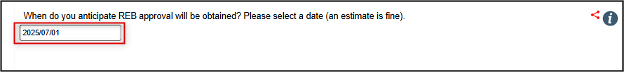
Enter the estimated date when you expect to have approval of your main REB application
- Reminder: although this date is an estimate, this date will drive future reminder notifications to renew or close your SOI
-
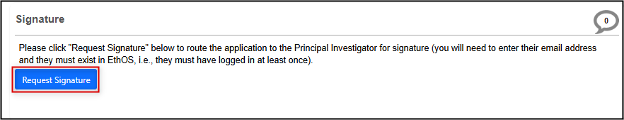
At the bottom of the page, click on the Request Signature button (or the Sign button if you are the PI)
Best practice tip: if you want the PI to provide feedback on the content, do not request the signature now. By assigning the PI role (in Step 6), they will have access to the form to provide any preliminary review, and you should incorporate this before you request their signature.
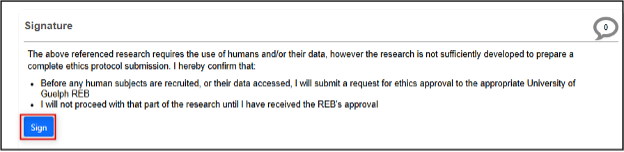
-
After the Statement of Intent is signed by the PI, it is automatically submitted; EthOS displays the Form Queued For Submission page and you will receive a confirmation email (this may take up to ten minutes and please check your junk folder)
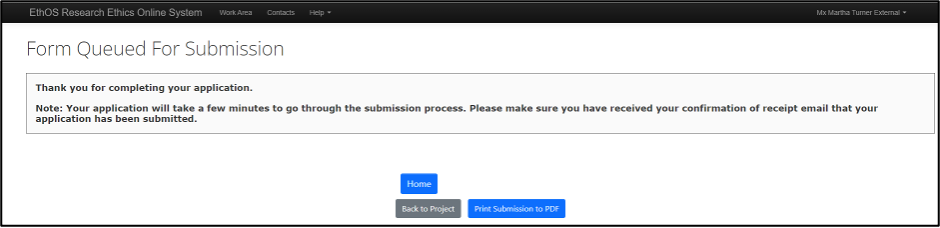
Once your SOI is submitted, the Ethics Office completes an administrative review on the application.
If modifications are required as part of the review process, please see Responding to SOI Modification Requests. Otherwise, upon approval, you receive an email and system notification with an attached Statement of Intent approval certificate.
Responding to SOI Modification Requests
When the Ethics Office completes a delegated review on the SOI, there may be minor modifications or suggestions prior to providing approval. In this event:
- You receive both an email and an EthOS notification to indicate that the SOI has been returned with a request for modifications
- The email includes a feedback letter outlining the suggested changes and the link to the SOI (which will now be in a status of Modifications Request SOI)
- After making the requested changes, the SOI must be re-signed by the PI upon which it is automatically resubmitted
To make requested changes to an SOI
-
Review the feedback letter in your email and/or system notification
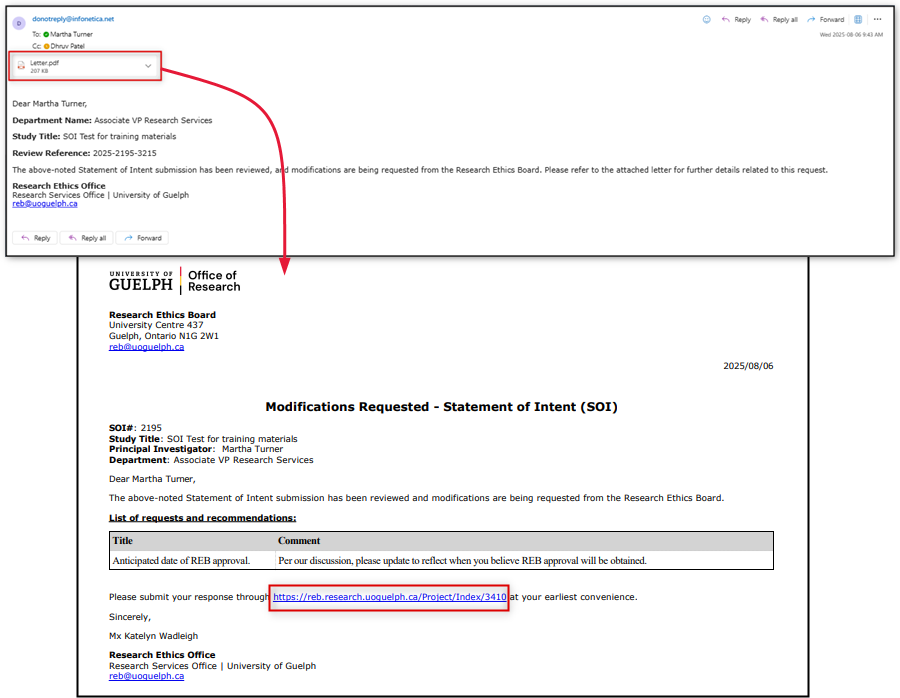
-
Follow the link from the feedback letter to open the application; notice the change in status to Modifications Request SOI
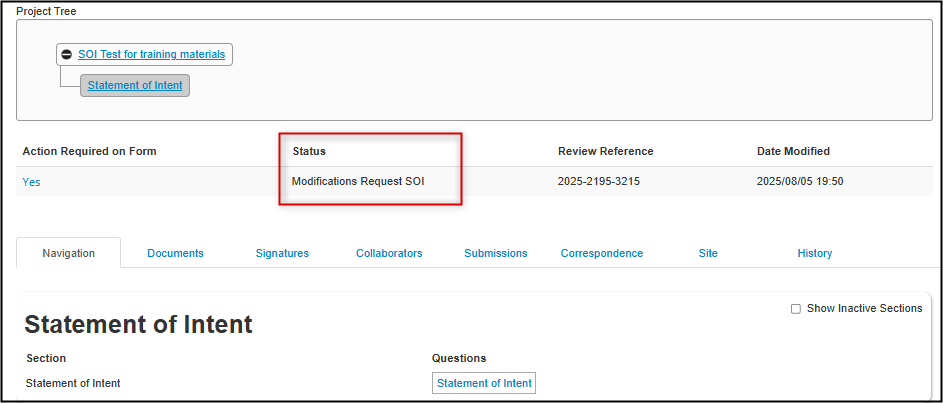
-
From the application’s main page, click on the Reviewer Comments tile to display the same list of comments also included in your feedback letter
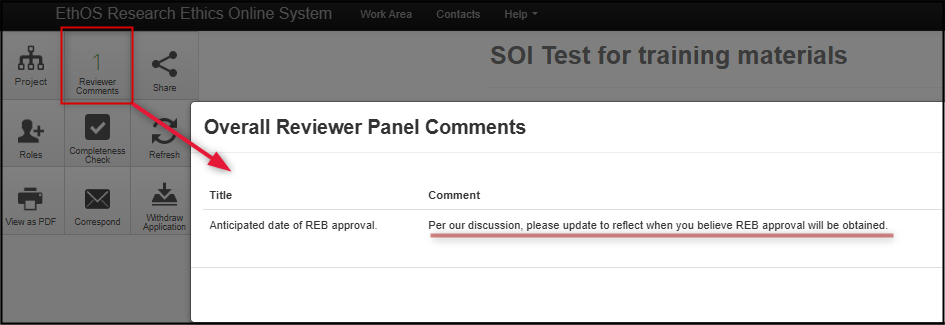
-
Click on any of the displayed comments to jump directly to its related question
-
Tip: if you need a reminder on the requested change or recommendation for this particular question, click on the Comment icon in the top right of the panel
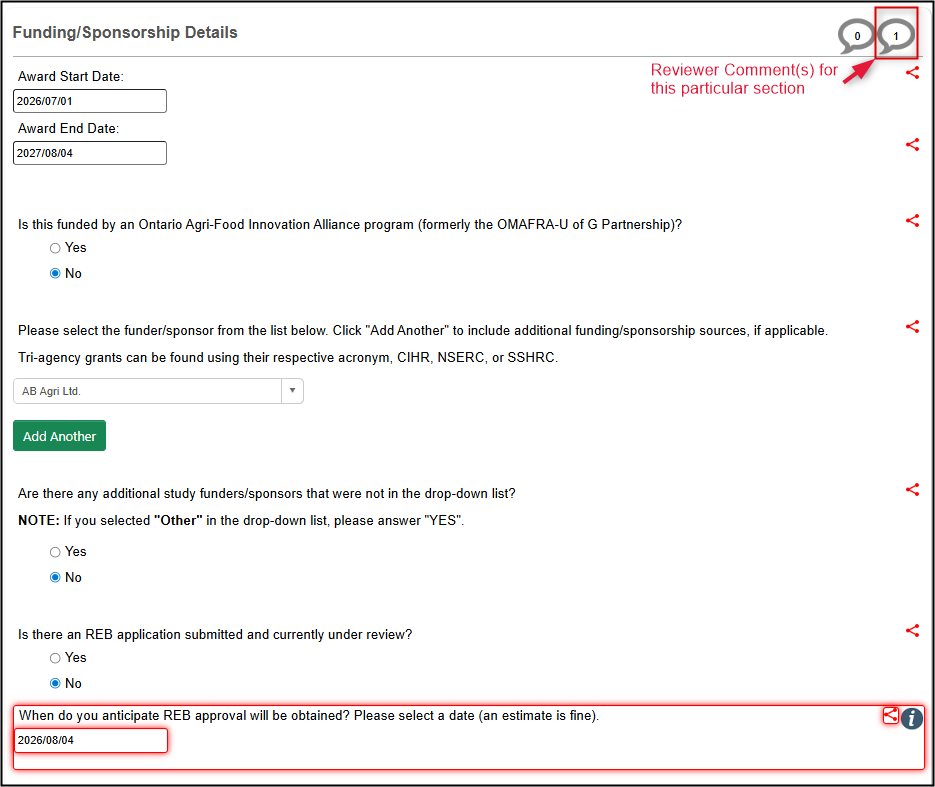
-
- Make the requested change(s) and click on the Save button
-
Optional: to see what changes you have made while addressing the feedback, click on the Compare with Previous Submission button in the top right corner to enter Track Changes mode
- Text in red is content you have deleted
- Text in green is content you have added
- To continue with your application, click on the Return to Edit Mode button

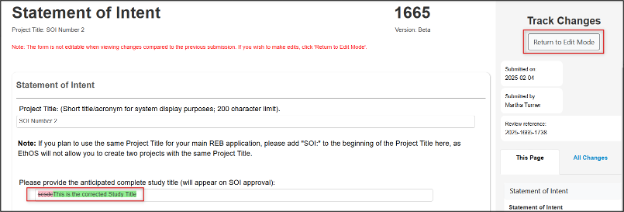
-
Continue making the requested changes by clicking the Reviewer Comments button and navigating to the related question, or by scrolling down the page and looking for a number in the next Comment icon
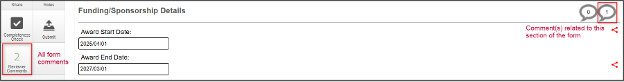
-
When all requested changes have been made, sign the SOI (if you are the PI) or request the PI signature
- This is the same procedure you followed in Steps 12 and 13 of the Create a Statement of Intent materials
Once the PI has re-signed, the SOI is automatically resubmitted to the Ethics Office and you receive an email confirmation and notification. Your SOI will continue in the administrative review process by the Ethics Office. Upon approval, you receive an email confirmation and system notification with an attached Statement of Intent approval certificate.
Renewing or Closing a Statement of Intent
When submitting the SOI, the anticipated approval date of the subsequent Main REB Application form is entered. This date should account for protocol preparation and review times (as can be best estimated at the time).
If the entered date passes without an REB approval, the SOI must be extended. Similarly, once REB approval is secured, researchers must officially close out the SOI as part of the grants and funding process. Both actions (renewals and closures) are facilitated by SOI Renewal/Closure forms within EthOS.
Reminder Alerts
To ensure compliance and avoid potential funding access issues, researchers now receive automated system notifications for upcoming SOI expiry:
- Two weeks before expiry
- On the day of expiry
Once you have renewed an SOI, future reminder alerts will be based on the amended anticipated approval date entered in the Renewal/Closure subform.
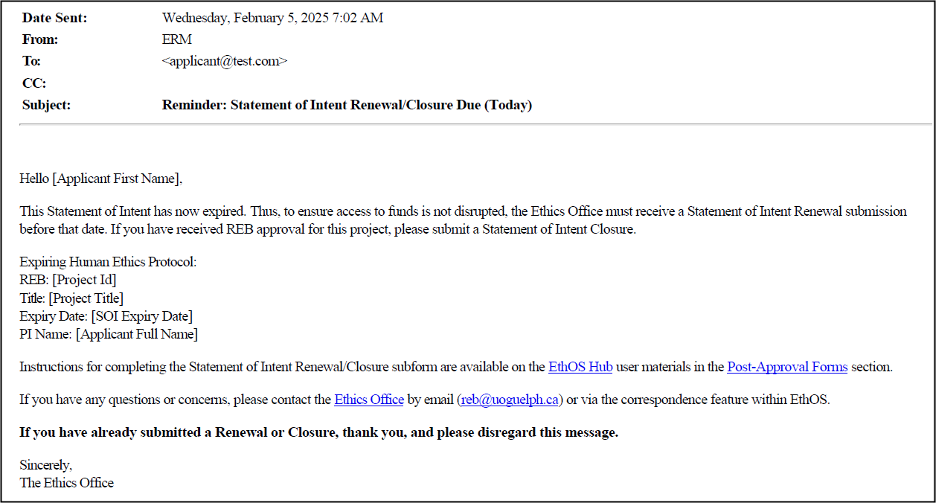
Example of a Reminder Alert
The reminder will contain header-level details about your SOI, including a link to access it directly in EthOS as well as a reminder of training materials. Due to the automated timing of alerts, it is possible that you may receive a reminder after submitting your renewal or closure. If so, please disregard the message.
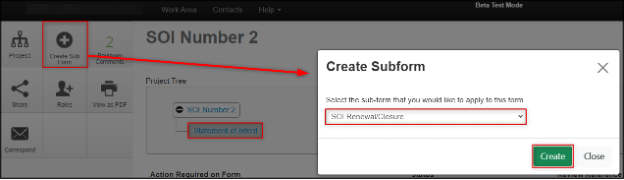
Creating the Renewal or Closure Subform
- Navigate to the SOI form (either from the expiry email, from your EthOS Notifications tile, or from your Project List)
- In the Project Tree section, ensure that your cursor is on the Statement of Intent ‘branch’ and click on the Create Sub Form button
- In the Create Subform pop-up window, the only option available is the SOI Renewal/Closure form
-
Click on the green Create button
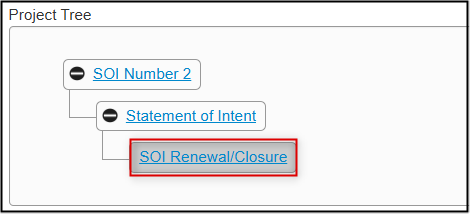
- EthOS displays a green banner message indicating that the subform was created successfully
-
In the Project Tree, the Statement of Intent branch expands to include the SOI Renewal/Closure form
The SOI Renewal/Closure form has two pages:
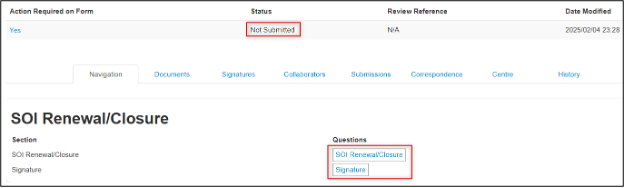
- Click on the SOI Renewal/Closure page
-
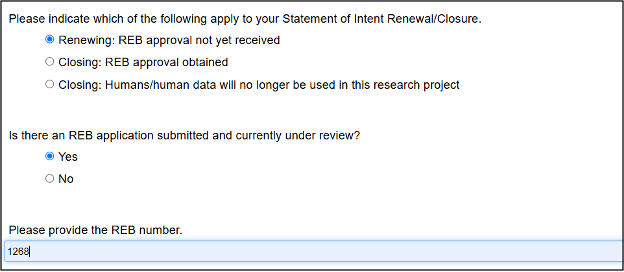
Review the header details (pulled in from your SOI) and scroll to the section of the form where you specify one of three options:
- Renewing – REB approval not yet received
- Closing – REB approval obtained
- Closing – Humans/human data will no longer be used in the research project
Continue from here based on your scenario below:
Renewals
-
Click on the Renewing option and answer the subsequent question about whether there is an REB application already under review, and if so, provide the REB number
-
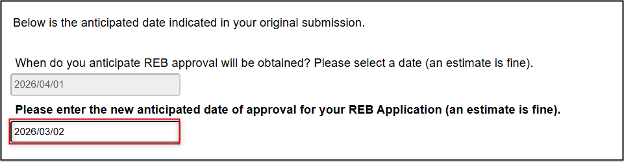
Enter the new anticipated (estimated) date of approval
- Note: future reminder alerts will now be based on this amended dat
- Scroll down to review the remainder of the form containing the award and funding details from your original SOI; you can edit these fields if needed
-
Navigate to the next page and sign the SOI Renewal (if you are the PI) or request the PI signature
- This is the same procedure you followed in Steps 12 and 13 of the Create a Statement of Intent materials
Once the PI has signed the SOI Renewal, it is automatically submitted to the Ethics Office and you receive an email confirmation and notification.
As with your original SOI, if the Ethics Office requires any changes prior to approving the renewal, your SOI Renewal/Closure form will be returned with a Modifications Requested SOI status. Follow the same steps as outlined in Responding to SOI Modification Requests.
Otherwise, upon approval you will receive an email and system notification including a revised Statement of Intent approval certificate showing the renewed date (and other changes you may have made). The Grants Team (Research Support Services or Agri-Food Alliance office) will also be notified of the SOI renewal.
Closures
-
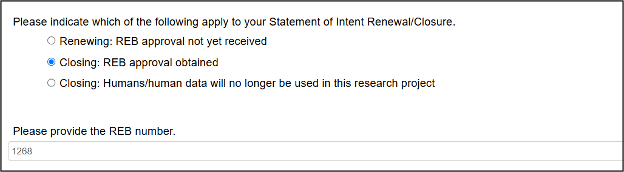
Click on the appropriate Closing option and enter the REB number of the approved main form application
- Scroll down to review the remainder of the form containing the award and funding details from your original SOI; you can edit these fields if needed
-
Navigate to the next page and sign the SOI Closure (if you are the PI) or request the PI signature
- This is the same procedure you followed in Steps 12 and 13 of the Create a Statement of Intent materials
Once the PI has signed the SOI Closure, it is automatically submitted to the Ethics Office and you receive an email confirmation and notification including the SOI Closure Notice. The Grants Team (Research Support Services or Agri-Food Alliance office) will also be notified of the SOI closure.