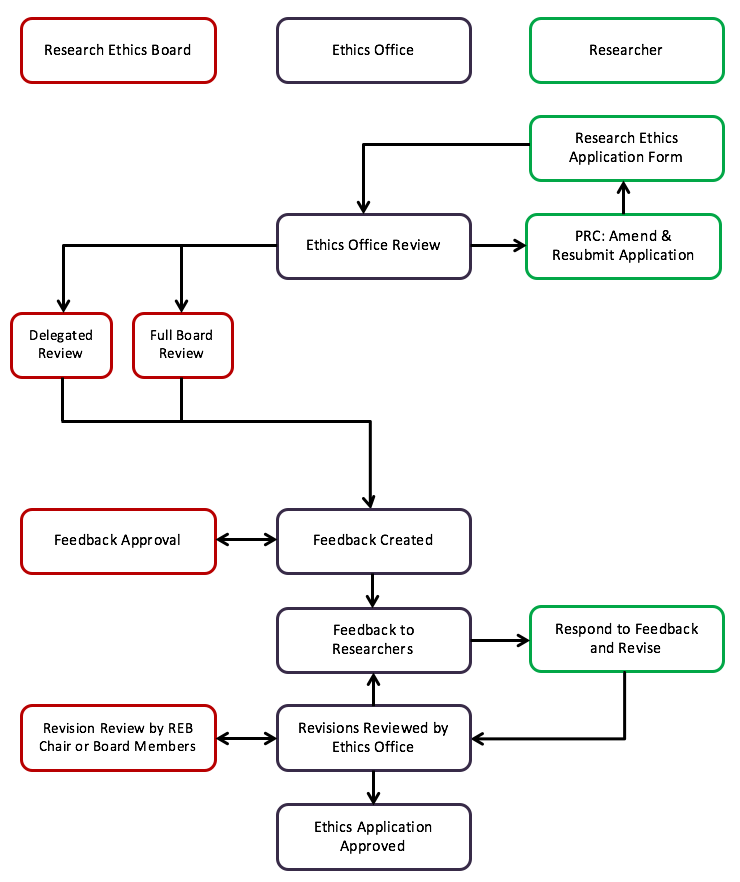
- Submission of application using EthOS as of July 25, 2024 or by email to reb@uoguelph.ca (if the application was already in flight prior to the launch of EthOS on July 25 2024). Please refer to the Interim Guidelines for more information.
- Ethics Coordinator:
- sets up e-file, and enters information in excel sheet (if received at reb@uoguelph.ca)
- completes the Pre-review for Clarification (PRC)
- Note: If changes are required as a result of the PRC, applicants who submitted through EthOS will update their applications in EthOS.
- Manager makes full board or delegated review decision. Full board decisions are confirmed by the Chair.
- Delegated review:
- If delegated, Ethics Office staff provides primary review of application and, in some cases, sends initial review plus all study documents to reviewers (REB members).
- Reviewers receive an email notifying them of the review with a subject line that provides a due date.
- Reviewers have one week to add comments and mark their review as done in EthOS or return to the Ethics Office by email (if this was an MS Word application)
- If a reviewer feels the application should be reviewed by the full board, they can notify the Ethics Office immediately and the application will be sent to the next full board meeting.
- The Ethics Office collates all reviews and sends final feedback to Chair, along with reviews.
- If Chair approves the feedback, this is sent to researcher (PI)
- Full board reviews:
- Ethics Office provides initial review – the ‘reviewers’ document
- Ethics Office’s review and all documentation are shared with REB members one week prior to the meeting.
- The submission will be presented by one REB member, after which discussion will open.
- The meeting may be attended by the researcher to answer questions from REB members.
- At meeting any member can comment on Ethics Office’s review – adding or removing items
- A final version is put to a vote at the meeting. Consensus is the desired outcome and is achieved in most cases. Abstentions or rejections will be minuted, but the REB member abstaining or voting against the motion can choose whether or not to be named in the minutes.
- The minutes containing the final feedback are approved by the Chair prior to the Ethics Office sending feedback to the researcher (PI).
- Modifications – these are sent by the researcher in response to REB feedback
- Delegated review – a reviewer can request to see modifications, or the Ethics Office can request reviewer comment on any modifications. Requests for modifications comments will be emailed to the reviewer.
- Full board review – it is determined at the original meeting who can approve the modifications. They can
- come back to the next full board meeting
- be reviewed by a sub-committee
- be reviewed by the Ethics Office and Chair.
- Amendments – these are sent in by the researcher to note a change to a protocol which is already approved.
- The Ethics Office will send to reviewers who did the initial review (if possible) if the content is beyond strictly administrative changes.
- Amendments will be considered by the full board if this is warranted by the level of risk.
- The documents will be emailed to reviewers as they are for regular reviews.